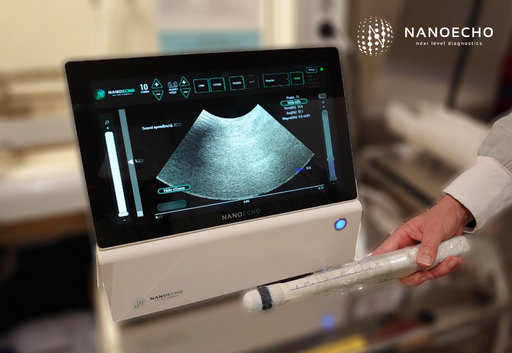
NanoEcho is developing a system with the aim to offer the healthcare a more precise, simple, and cost-effective rectal cancer diagnosis. The prototype system used in the ongoing investigator-led clinical development studies has now been replaced with a system intended to meet market requirements. The first examination on surgically removed rectal cancer tissue with this system has now been performed.
In December, the detailed product development phase was completed. This resulted in a system developed with the aim to meet the market’s requirements, including both the authorities’ and customers’ requirements. The product development phase has now moved into an integration and evaluation phase.
The integration work is proceeding according to plan. We assess that the new system’s performance is adequate to replace the prototype system used in the ongoing clinical development studies. The first examination of surgically removed rectal cancer tissue has now been performed. We aim to increase the quality of collected clinical data and obtain valuable user information contributing into the evaluation of the system.
The fact that we have already replaced the prototype system with the new system in our investigator-led clinical development studies is the result of focused integration work carried out by our dedicated, experienced team. While we gather valuable feedback from the use of the system, we will continue with further integration work, comments CEO Linda Persson.
For further information, please contact:
Kristina Hallström, CMO & CCO
email: ir@nanoecho.se
NanoEcho develops a new technology for clearer diagnostics of, in the first indication, rectal cancer. The imaging technology is based on a new medical approach where nanotechnology is used in combination with modern patented ultrasound technology. The images that are generated are intended to facilitate differentiation between healthy and diseased tissue and at the same time determine the location of the cancer tissue more precisely. The aim is to provide more precise, simple, and cost-effective diagnosis of cancers and other diseases. With clearer diagnostics, the company wants to assist treating physicians with better guidance for more personalised treatment. Both the quality of life of the patients and their chance of survival can improve after treatment, with reduced treatment costs. www.nanoecho.se