Vision and strategy
NanoEcho is a company developing a new diagnostic method to determine whether early rectal cancer has spread to nearby lymph nodes – a method that has the potential to generate significant health economic gains.
The vision is that all rectal cancer patients will receive a correct diagnosis and thus optimal, individually tailored treatment.
The strategy includes NanoEcho introducing a new method for improved diagnostics of early rectal cancer and applying for authority approval for its imaging system during 2028. The system shall have the functionality to meet customers’ needs and create health economic benefits.
The ambition is for the examination to become clinical practice for rectal cancer patients.
A piece that is missing in today’s healthcare
Today, clinical practice for rectal cancer diagnosis is based on tissue analysis, computed tomography of the chest and magnetic resonance imaging (MRI). These examinations are the basis for the selection of treatment and constitute clinical practice. Unfortunately, there is a lack of a method to reliably map the spread of rectal cancer to nearby lymph nodes, an important piece of the puzzle for choosing optimal treatment. NanoEcho’s goal is to fill this global gap in today’s healthcare.
The road to the goal
Product development
NanoEcho’s imaging device is fully developed and meets the requirements of both customers and regulatory authorities for medical devices. The system is now being evaluated in a clinical study.
Clinical focus
Development of the diagnostic method and the clinical path forward takes place in close collaboration with doctors in rectal surgery and radiology, preferably at university hospitals.
Market
Focus is market approval in Europe, with an initial focus on Sweden. A health economic model has been developed to be used as a basis for pricing.
Attractive business model
The business model is based on the “razer-blade model”, the customer pays per treatment. This creates an ongoing revenue stream throughout the system’s life cycle. Alternative business models may be evaluated.
Sales
The plan is to establish sales through partnerships with distributors/medical technology companies that already sell imaging systems today.
Value-creating milestones
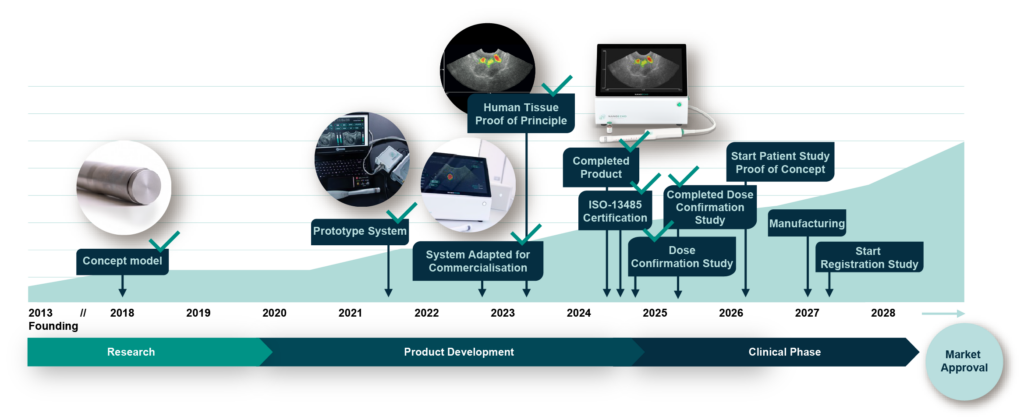
Disclaimer: The present plan is based on NanoEcho being able to raise the necessary capital.
Our history
NanoEcho was founded by a research group at the Department of Biomedical Engineering at Lund University. The company was registered in 2013, when the first patent application was submitted, the focus was research. In 2019, NanoEcho raised capital and in 2020, the current organisation began to be established under the leadership of CEO Linda Persson.
Future potential
In the next step, there are good opportunities to further development of NanoEcho’s equipment and method for other clinical use.
Prostate cancer
Prostate cancer is one of the most common cancers. Our probe will, after minor adjustments, also be able to be used to image the relevant areas around the prostate gland. We estimate that clinical studies can start two to three years after the technical development of the equipment has started.
Atherosclerosis
Atherosclerosis means that there is an injury inside a blood vessel that leads to deposits (plaque) that can loosen and cause a blood clot. Plaque should be able to attract nanoparticles via immune cells, which could be detected with our method. We assess that clinical studies can start approximately 4-6 years after the technical development has started.
Stem cell therapy
Stem cell therapy is based on adding stem cells that stimulate the rebuilding of the damaged organ. A possible future application is to monitor the process by preparing the stem cells with nanoparticles.The area is at the research stage.
