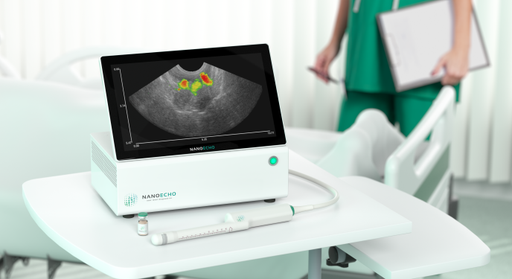
NanoEcho has now successfully completed the detailed product development phase. The work has resulted in a system that has been developed to meet both the authorities’ and customers’ requirements. This system consists of parts manufactured by the company’s selected partners and key suppliers, Vermon and us4us. The system is now on site at the company’s premises in Lund, ready for integration and evaluation.
The detailed product development phase is now complete and the company’s product development work is moving into an integration and evaluation phase. In this phase, all the parts of the system, that the company has developed together with its partners, are assembled and the system software will be developed.
Once the integration work is completed, the system will be evaluated with regard to authority and customer requirements. The company’s ambition is to replace the prototype system currently used in the ongoing investigator-led clinical development studies with this new system in 2023.
“We have had our sights set on this milestone all year, and today I am extremely proud that we now have our system assembled on site here in Lund. Both our team and our partners have done a very impressive job, developing and producing this system to meet highly set medical technical requirements in such a short time. When we replace the prototype system with this system in the ongoing clinical development studies, the goal is to increase the quality of the data we obtain,” says Linda Persson, CEO of NanoEcho.
For further information, please contact:
Kristina Hallström, CMO & CCO
email: ir@nanoecho.se
NanoEcho develops a new technology for clearer diagnostics of, in the first indication, rectal cancer. The imaging technology is based on a new medical approach where nanotechnology is used in combination with modern ultrasound technology. The images that are generated are intended to facilitate differentiation between healthy and diseased tissue and at the same time determine the location of the cancer tissue more precisely. The aim is to provide more precise, simple, and cost-effective diagnosis of cancers and other diseases. With clearer diagnostics, the company wants to assist treating physicians with better guidance for more personalised treatment. Both the quality of life of the patients and their chance of survival can improve after treatment, with reduced treatment costs. www.nanoecho.se